Over the decades, in vitro fertilization (IVF) has evolved from an experimental procedure to a standard treatment for infertility, contributing to the birth of over 10 million babies worldwide. However, like any medical intervention, fertility treatments carry inherent risks.
IVF procedures do not occur in a sterile environment. Despite meticulous environmental engineering and sterile techniques, the reality is that biological materials used in IVF, such as sperm and eggs, are prone to contamination with viruses, fungi, and bacteria. This contamination can have devastating consequences, leading to the demise of valuable embryos and ultimately resulting in a wasted cycle for the patient.
Moreover, the transmission of microorganisms into patients through contaminated embryo culture media poses another significant risk. This can lead to decreased endometrial receptivity or, in severe cases, infection transmission.
Studies have indicated that the incidence of overt bacterial contamination in IVF cycles is between 0.35% and 0.86%. With over 400,000 IVF cycles performed in the United States in 2021, the importance of robust infection prevention measures cannot be overstated.
To mitigate the risks associated with handling biological materials in IVF procedures, comprehensive measures are necessary. In a article published in 2000 in Human Reproduction, Dr Gianaroli said vaccination of laboratory personnel against hepatitis B and other viral diseases is crucial, providing an additional layer of protection against potential occupational hazards. Routine screening of patients and gamete donors for infectious diseases before treatment or cryopreservation is imperative to identify and mitigate transmission risks effectively.
Furthermore, he also said protective measures within the IVF lab itself are paramount to maintaining aseptic conditions for gametes and embryos throughout procedures. This includes the use of laboratory clothing, non-toxic gloves, and masks to minimize the risk of exposure to infectious agents.
He suggested to give special attention to handling cryogenic materials, with eye and face protection being essential safeguards against potential hazards. Controlled environments are created using vertical laminar-flow benches, mechanical pipetting devices, and fume hoods to ensure sterile procedures.
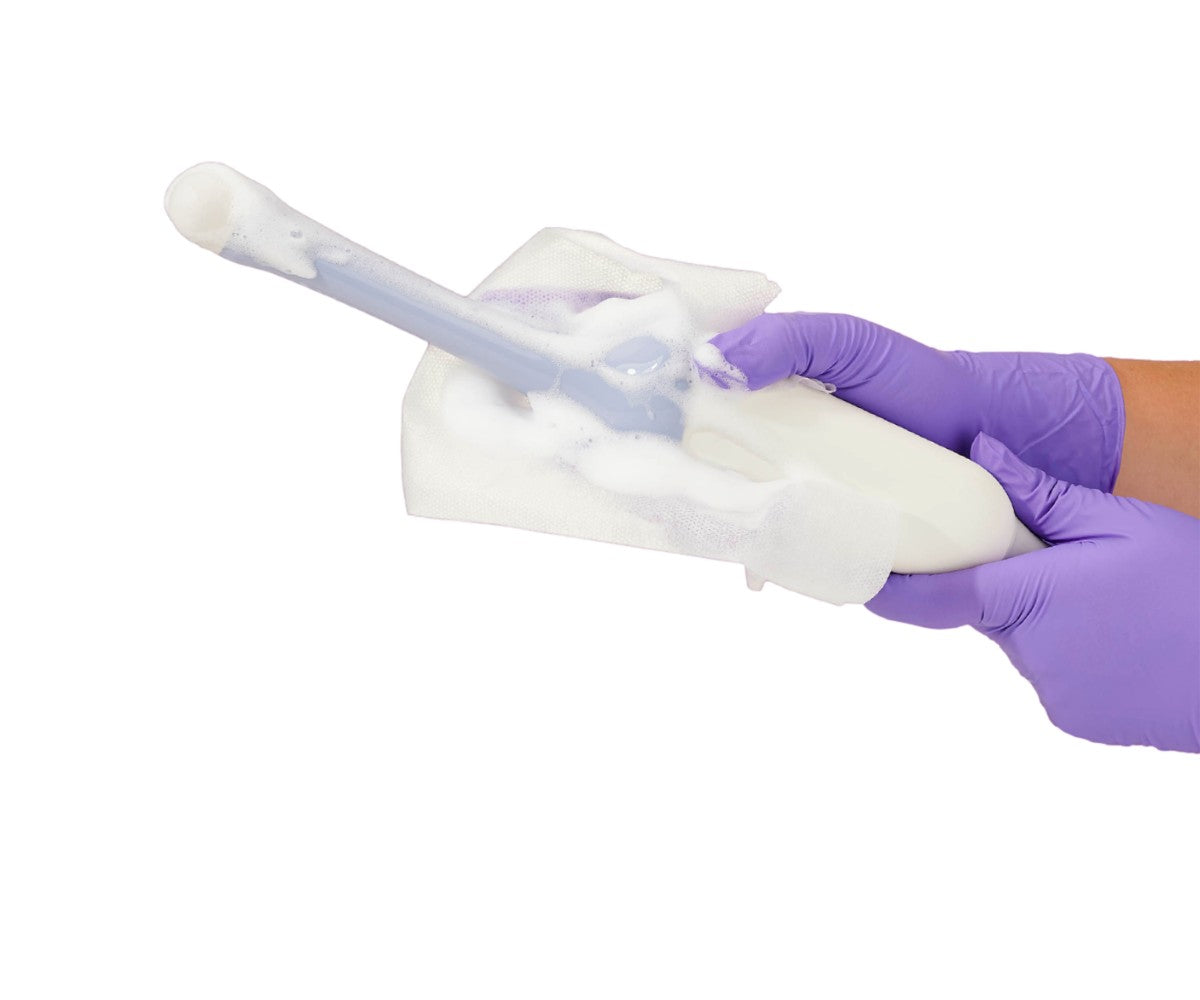
Tristel ULT leverages the advanced chlorine dioxide (ClO2) technology unique to Tristel's suite of offerings. This sophisticated system produces chlorine dioxide foam through the combination of two distinct solutions - the Base Solution and the Activator Solution. These components are meticulously contained within separate vessels, unified within an elegantly designed dispenser unit known as the "pump."
Moreover, chlorine dioxide operates as a potent oxidizing biocide, effectively neutralizing microorganisms by breaching their cellular defenses and disrupting essential nutrient transport mechanisms by targeting protein synthesis. This mechanism is crucially effective irrespective of the organism's metabolic state, rendering it highly efficient against both dormant entities and spores.
Regardless of adopting stringent measures such as these, the risk of cross-contamination may exist during cryopreservation procedures. While there is currently no documented evidence of such incidents, it is strongly advised to implement precautionary steps to mitigate any potential risks.
Overall, infection prevention in IVF labs requires a multi-faceted approach. Through the adoption of these strategies, IVF clinics can minimize the risks associated with handling biological materials and ensure the safety and success of fertility treatments for patients.